FreMon Scientific distributors, investors, staff, Board of Directors and specialists gathered at the San Diego Blood Bank Thursday Sept 5th 2019 to see ZipThaw202 and ZipSleeve in action.
SDBB experts demonstrated ZipThaw and ZipSleeve, and reviewed the results from the from FDA 510(k) submission tests. Spoiler alert: ZipThaw performed as well as — and better than — the predicate plasma thawing water bath device. ZipThaw demonstrated superior results in several crucial coagulation factors. This gives us great confidence as we head to an FDA submission. ZipThaw currently has CE Mark and ISO certification.

SDBB technician demonstrates a full ZipSleeve inserted into ZipThaw, as distributors and investors look on.
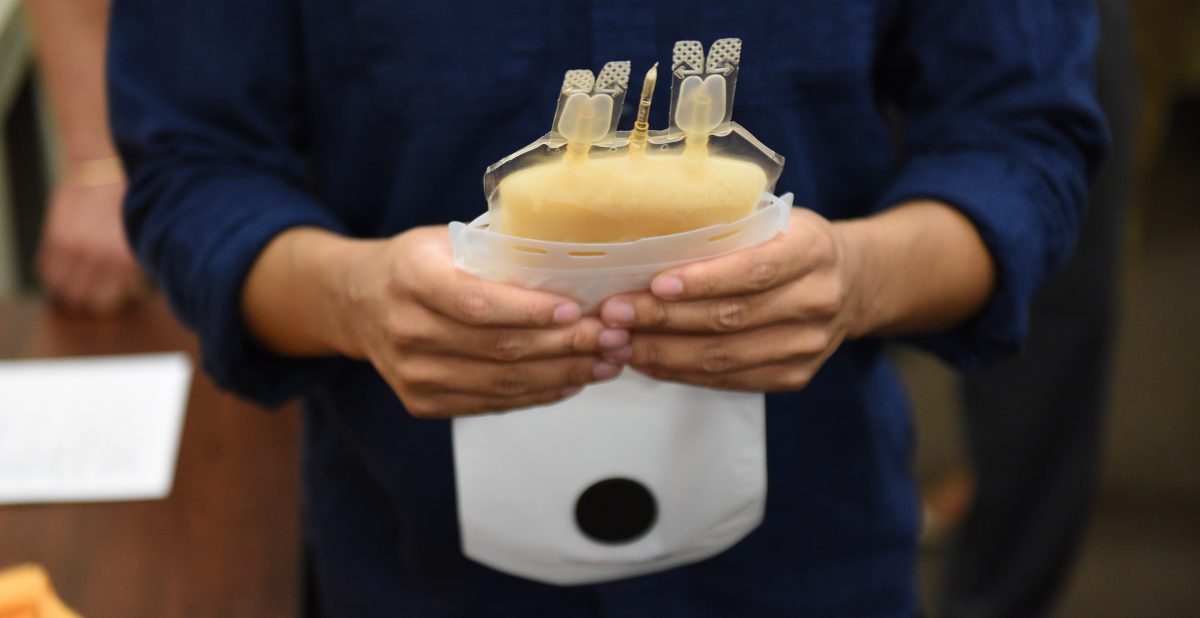
At the San Diego Blood Bank, lab technicians demonstrated ZipThaw’s capability to thaw demo plasma — in this case, frozen apple juice — and how to operate the device with its simple touchscreen controls and integrated barcode scanner.

Plasma bag ready for ZipSleeve, with a ZipThaw chamber open.
Participants observed the full life cycle, asked probing questions, paused and resumed the thawing process, took temperatures, touched the device and had fun.
After the initial demonstration, SDBB technicians conducted a full-scale training program for distributors representing Africa, the Middle East, Germany, the UK, Ireland and the USA.

Sarah Betteley of Deva Medical UK and Ireland trains with ZipThaw and ZipSleeve. Mike Miller from SpringTide, a USA distributor, looks on.

Distributors, investors and co-founder Moni Shavit observe ZipThaw.

Chris Barkey, of Barkey Medical, trains on ZipThaw.

Co-Founder Fred Thacher demonstrates ZipSleeve to American distributors and investors.
We also enjoyed an in-depth presentation by SDBB manager Will Davey and then Dr. Dzung Le, Chief of Coagulation Medicine at the UC San Diego School of Medicine, as he explained the FDA testing results in detail.

Dr. Le presents testing results.

Will Davey of SDBB presents ZipThaw to the group.
SDBB Manager Will Davey discussed ZipThaw’s ability to dramatically improve clinicians abilities to quickly and safely deliver Fresh Frozen Plasma at optimal temperature to patients in need of transfusions.
Improving patient care and literally saving lives energized the room as we observed the next generation of plasma thawing unfold before our eyes.

Founders Fred Thacher and Moni Shavit introduce Dr. Le of UC San Diego School of Medicine