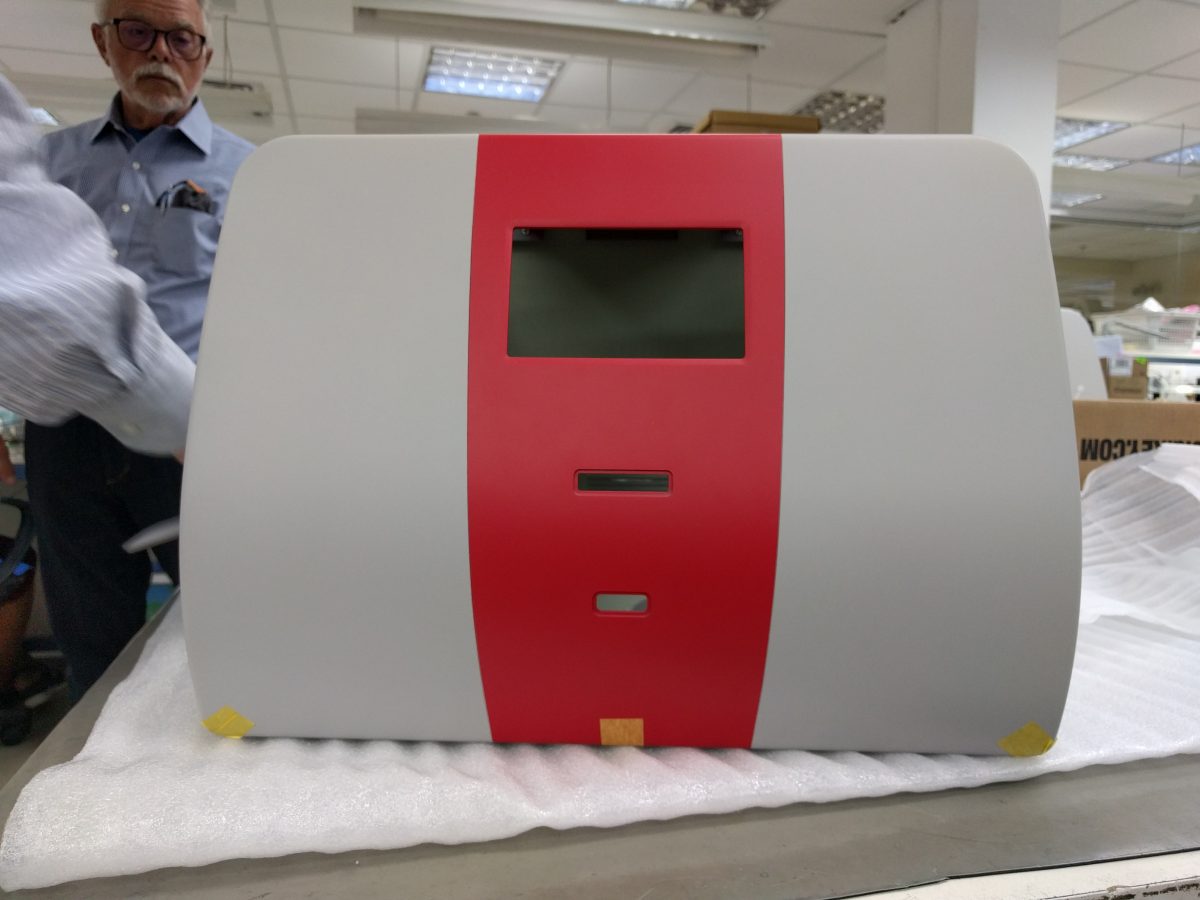
As a reminder, the CE Mark is for the European Union. It’s their version of the FDA.
This last test is termed a destructive test designed to actually destroy the medical device in order to determine it’s stability and safety under the most extreme conditions. In the first such round ZipThaw202 did well with a few electronic leaks which required simply switching out metal retaining screws with plastic and shielding the touch screen.
Moni Shavit supervised the alterations with Sysmop. We are confident of FDA approval and CE Mark issuance.
Today ZipThaw202 is so close to being real. The Board of Directors will meet this week to authorize contracting with an engineering firm in Israel to commence production engineering. Sales will begin 10 months from today subject to funding success.
Moving forward means we must complete funding. Please contact me to learn more. Risk has been substantially reduced with three new events:
1.) ZipThaw202 passing FDA functional and destructive testing,
2.)FDA and CE Mark submissions within 14 days, and
3.) Seven new USA and one UK based distributors in negotiations.
Moni and I deeply appreciate the guidance and support from you. Without you ZipThaw202’s advanced next generation blood, plasma, stem cell and tissue warming and thawing technology would not be possible. To bring it to market we need just a bit more. Please dig deeper to help close this round. As always, Moni and I are directly available.
With respect, Fred
Frederick Thacher
CEO
FreMon Scientific, Inc.
Fred@fremonscientific.com