Fred Thacher, co-founder of FreMon Scientific, attended the Precision Medicine World Conference this week in Palo Alto.
FreMon Scientific
FreMon ScientificFeatured
Touring Genesis BPS
Thanks to Mark Rosenfeld for the visit and tour of the state-of-the-art Genesis BPS facilities in New Jersey.
ZipThaw continues testing in San Diego
Over the past week we’ve continued testing ZipThaw and ZipSleeve at the San Diego Blood Bank.
Making strides at the SD Blood Bank
Today at the San Diego Blood Bank we’ve continued testing ZipThaw for FDA review.
ZipThaw FDA testing underway!
FDA testing of the ZipThaw blood and plasma product thawing device is now underway in San Diego. Here are outtakes from the lab.
ZipThaw with Deva Medical in the UK
Deva Medical is the first international medical device distributor to show ZipThaw and ZipSleeve at a conference, in this case, the Stem Cell Conference in Cambridge, England.
ZipThaw ships to San Diego Blood Bank
Today FreMon Scientific co-founder Moni Shavint packed shipped a ZipThaw202 and ZipSleeves for delivery to the San Diego Blood Bank where it will undergo final testing.
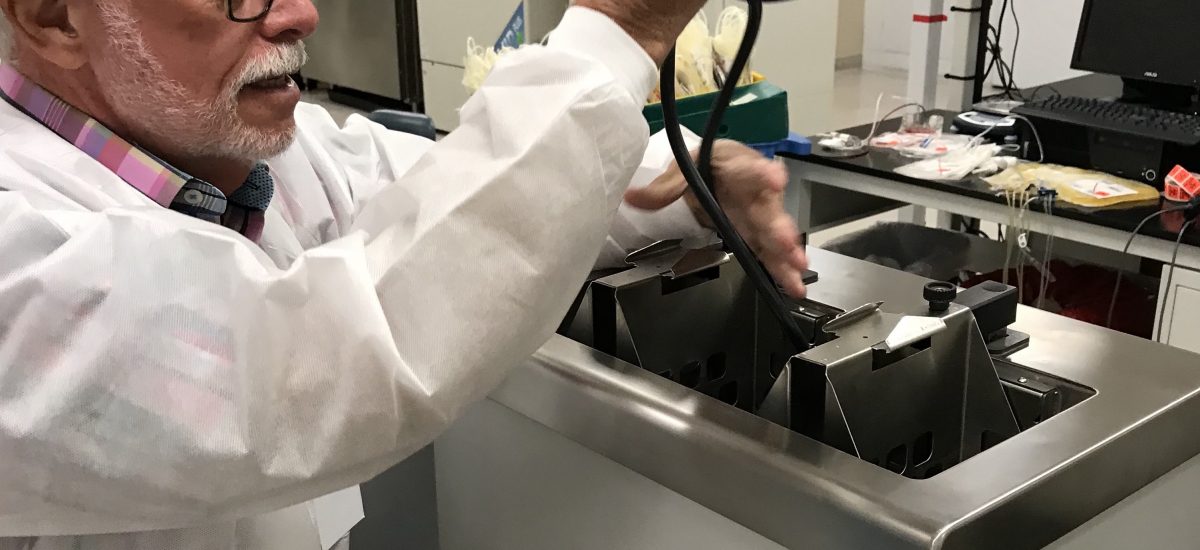
FDA Testing at UCSD is On!
Today FreMon Scientific co-founder and CEO Fred Thacher went to the UCSD labs to supervise FDA testing of ZipThaw. Please see the photos below
ZipThaw on the Assembly Line
Today we’re constructing ZipThaws on the assembly line as we prepare for distribution.
EU review of ZipThaw in Israel
We’re having a busy week in Israel.
UCSD testing ZipThaw for FDA
We’re thrilled to partner with the University of California, San Diego for FDA testing of ZipThaw.
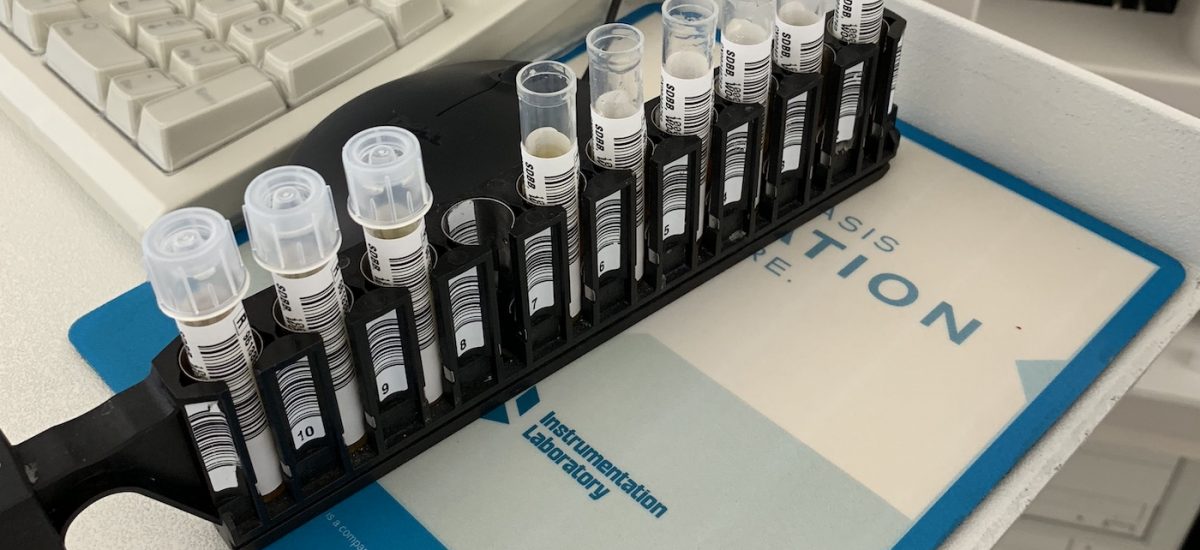
SDBB Predicate Tests Advancing
Today Fred Thacher, CEO and co-founder of FreMon Scientific, oversaw continued predicate testing of fresh frozen plasma devices at the San Diego Blood Bank.
FMS at AABB 2018 Boston
FMS, led by co-founder Fred Thacher, attends the 2018 AABB in Boston this week. (more…)
Predicate device setup at the SDBB
FDA testing of ZipThaw requires us to set up a predicate plasma thawing device. We’re thrilled to be partnering with the San Diego Blood Bank on this critical data gathering and benchmarking phase.
Production preparation
FreMon Scientific, in preparation for a second European Union CE Mark medical device audit, is in the pre-production assembly line development stage.
ZipThaw Production-Ready Chassis
We’re entering a bold new phase.
San Diego Blood Bank to test ZipThaw
San Diego Blood Bank has agreed to test ZipThaw and ZipSleeve devices.
CE Mark as a Medical Device
San Diego Blood Bank
We’re grateful for the San Diego Blood Bank’s guidance with ZipThaw.
FDA approval in process
FreMon Scientific filed a 510(k) application with the FDA for ZipThaw202 and ZipSleeve.