FreMon Scientific is now all set up at Medlab 2020 Dubai. If you’re in the region, please join us to see ZipThaw and ZipSleeve in action.
FreMon Scientific
FreMon ScientificFeatured
ZipThaw at Dubai:MedLab
We’re not sitting still at FreMon Scientific.
Tom Rosenbloom hired as President
FreMon Scientific announces the appointment of Thomas A. Rosenbloom as President and Chief Legal Officer.
See ZipThaw at Arab Health: Innovation Hub
At Arab Health 2020, the FreMon Scientific team has been meeting hundreds of people interested in learning more about ZipThaw and ZipSleeve.
(more…)
Behind the Scenes with ZipThaw
Last week Will Davey, VP of Product Management and Design, worked with a talented crew of producers to craft a series of instructional videos for how to use ZipThaw and ZipSleeve.
Training for Dubai
ZipThaw202 and ZipSleeve, FDA cleared, are on their way to the Arab Health and Arab Lab trade shows in Dubai January 26-February 5.
To prepare for presentations to distributors and buyers, William Davey, FreMon Scientific VP for Product Management and Design, trains Jim Pilkington, Rosemary O’Toole, Amy Pilkington and Fred Thacher on how to use ZipThaw and ZipSleeve.

Training is being conducted at the San Diego Blood Bank.
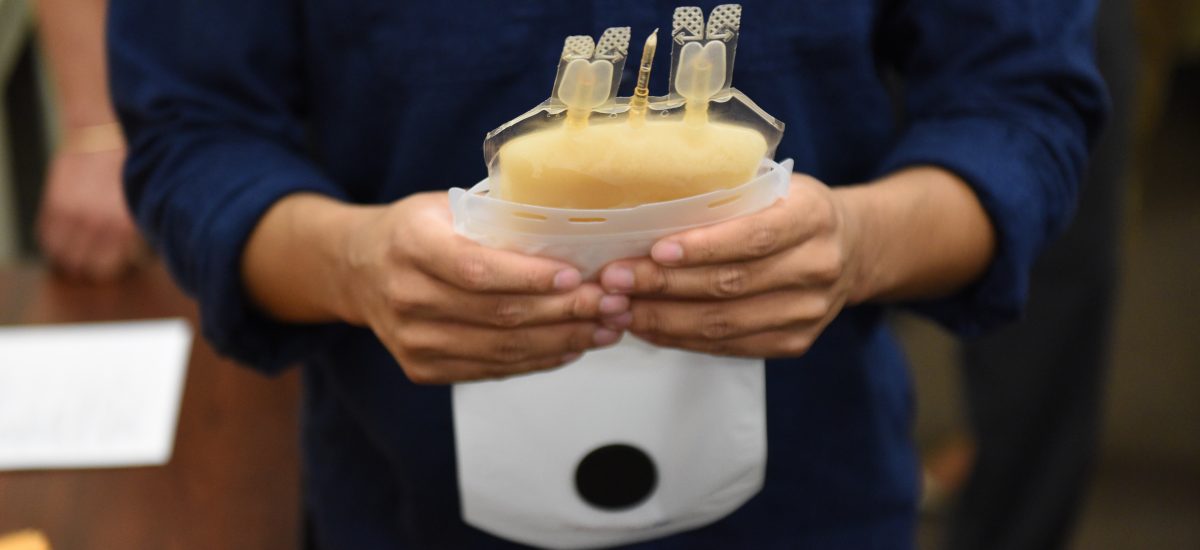
Notice the ZipSleeve patented disposable on the bench below Will’s left elbow. ZipSleeve is an entirely new and advanced way to precisely thaw plasma with little fear of cross contamination.

Dr. Farideh Bischoff, CEO of FreMon Scientific is overseeing all aspects of final commercial manufacturing and production. Today she’s also receiving a refresher course from Will Davey.
ZipThaw is amazingly easy to use and reduces any risks of cross contamination by using the ZipSleeve disposable designed for 8 uses. ZipSleeve makes certain every plasma sample is thawed safely and rapidly to the exact temperature for patient use. See you in Dubai!
ZipThaw Receives FDA Clearance: Press Release
ZipThaw receives FDA clearance for clinical use. Read the official press release: https://www.prnewswire.com/news-releases/zipthaw-receives-fda-clearance-for-clinical-use-300980923.html?tc=eml_cleartime
ZipThaw: Made in Minnesota
FreMon Scientific is pleased to announce our partnership with Minnetronix Medical (https://minnetronixmedical.com/) of St Paul, Minnesota to begin the manufacturing and commercial production of ZipThaw, the world’s first dry, portable, precise Fresh Frozen Plasma thawing medical device. While ZipThaw is new to the market, Minnetronix has been creating life-saving medical devices since 1996. They are a pioneering medtech company that brings together extraordinary technology, skills and passion to accelerate the delivery of medical devices and other patient-focused treatments.
Minnetronix has an operating philosophy of not just making things, but making things better — for customers, for patients and for healthcare overall. Their focus is a perfect fit with FreMon Scientific’s dedication to improving and saving lives. Minnetronix deploys a certified, best-in-class quality management system for electronic and electromechanical medical devices, and they use a proprietary product development process designed for consistency, speed and agility.
Most critically, Minnetronix has undergone seven successful FDA Quality System Inspection Technique (QSIT) inspections with No Action Indicated. In a typical year, their QMS is subjected to more than 30 audits by regulators and customers. We’re privileged to partner with such an innovative quality-focused and dedicated team.
ZipThaw and ZipSleeve are manufactured in the USA, with CE Mark, ISO13485 certification, UL listing, and FDA cleared. To purchase ZipThaw and ZipSleeve, please contact our distributors: https://fremonscientific.com/distributors/
ZipSleeve: Made in Massachusetts
FreMon Scientific is proud to announce it’s agreement with Boyd Technologies (http://boydtech.com/) in Lee, Massachusetts for the manufacturing and production of ZipSleeve.
FDA clears ZipThaw for clinical use
Ready across the EU
Last month FreMon Scientific successfully passed the annual ISO 13485 quality audit and CE Mark renewal. This means ZipThaw and ZipSleeve are approved for clinical use to thaw and warm plasma in all CE Mark countries — across Europe including the UK, Iceland and Turkey.
ZipThaw is a portable, dry plasma thawer with precise thawing capabilities. To purchase ZipThaw and ZipSleeves from FreMon Scientific’s European distributors, please visit https://fremonscientific.com/distributors/
Made in the USA
ZipThaw manufacturing kicks off in St. Paul, Minnesota.
Drawing crowds at the AABB
More than 300 attendees have come to booth #443 at the AABB 2019 annual conference to learn about ZipThaw202 and ZipSleeve.
ZipThaw at the 2019 AABB
Today the AABB welcomes FreMon Scientific Team to their annual conference, this year in San Antonio Texas.
See ZipThaw at booth #443 AABB San Antonio
Come to booth #443 at the 2019 AABB in San Antonio, TX to see ZipThaw and meet the FreMon Scientific team.
ZipThaw in Seville
Hello from Seville, Spain! (more…)
Watch the ZipThaw Distributor and Manufacturer Presentations
We worked with a talented videographer to capture the ZipThaw and ZipSleeve distributor and manufacturer presentations at the San Diego launch event. Watch them here:
Distributors and Manufacturers Present ZipThaw and ZipSleeve Sales Plan
Distributors and manufacturers presented their rollout plans for ZipThaw202 and ZipSleeve at the FreMon Scientific San Diego Launch Event.
ZipThaw and ZipSleeve Launches at the San Diego Blood Bank
FreMon Scientific distributors, investors, staff, Board of Directors and specialists gathered at the San Diego Blood Bank Thursday Sept 5th 2019 to see ZipThaw202 and ZipSleeve in action.